Category Archives: Science
Pandas, Kitzmiller, and the frozen frog fallacy
By Paul Braterman
January 4, 2023 13:00 MST
Paul Braterman is Professor Emeritus at the University of North Texas and Honorary Senior Research Fellow in Chemistry at the University of Glasgow
This Kitzmas was different. For the first time, the Discovery Institute allowed the anniversary of Kitzmiller v. Dover Area School District to pass without complaining about the verdict. Perhaps they are hoping that we will forget about the incredible badness of the text that they were trying to foist on the District’s students. Of Pandas and People is carefully constructed to be as misleading as possible, and we shouldn’t let them forget this, as long as contributors and advisers responsible for it remain in position within the Discovery Institute1, while the Institute continues to promote works such as Denton’s Evolution – A Theory in Crisis, that perpetuate the same elementary errors of logic. But first, an apparent digression. When students first come across the use of molecular or DNA sequencing in constructing phylogenetic trees, they are sometimes puzzled. They have been told that mammals are descended from fish by way of amphibians. Therefore, as a matter of common sense, they might expect that frogs should be closer to fish in evolutionary terms than we are. This is another example of the Evolution as Progress error. While amniotes have progressed through synapsid to mammal to humans, the pinnacle of creation, the frog has remained a lowly frog and should, therefore, be closer to the common ancestor, as if the ineluctable processes of molecular mutation had somehow been suspended. We might call this the “frozen frog fallacy.”
At this point, a Table like the one shown below might help:
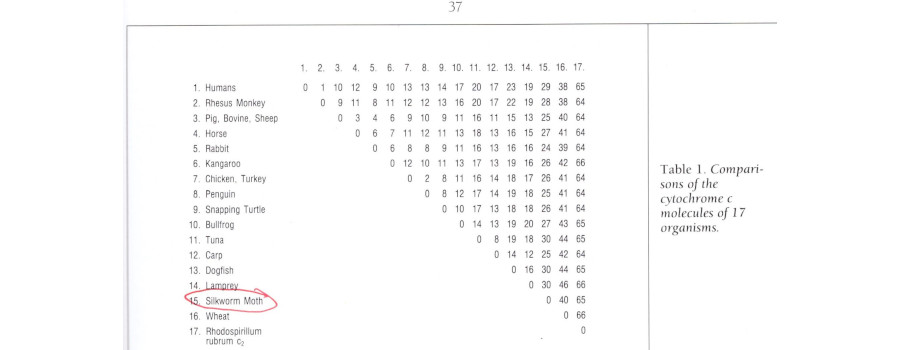
All the multicellular organisms shown are, apart from small random fluctuations, at the same distance from the bacterium, as expected if they share a common ancestor distinct from bacteria, which have of course, independently, been accumulating their own set of changes.
All the animals shown are, apart from small random fluctuations, at the same distance from wheat, as expected if they share a common ancestor distinct from plants. And the relative number of mutations shows that the split between plants and animals is more recent than the split between multicellular organisms and bacteria. More recent yet is the split between fish and tetrapods, leaving all tetrapods (including you and me, and of course present-day frogs) at the same distance from the fish. And so on. Most tellingly, humans share a common ancestor with monkeys, more recent than their common ancestor with non-simian mammals. There is a lot more detail in the Table, for example about how to birds relate to reptiles, and how the different orders of mammal relate to each other. And of course the construction of a phylogenetic tree is based on the specific differences found, rather than the overall number.
There are now numerous published studies of the phylogenetic relationships revealed by Cytochrome C, to say nothing of the vast recent literature using numerous molecular and morphological traits to develop detailed high-resolution phylogenies, and to explore the limitations of the concept of a unique phylogeny. What is interesting about the particular Table I have quoted is its origin, and the uses that its authors make of it.
This brings us back to our original theme. The Table actually comes from Of Pandas and People, 2nd edition, 1993, which by the time of the trial had gone through five printings. The book does not give a reference to the source of the data, but much (not all) of the information can be found in a classic 1967 paper [2], which also explains the reasoning behind the method, and critically examines the assumptions made. So there is no excuse for what the book does next, which is to repeatedly assert that the data refute claims of common ancestry:
one might expect analysis to reveal that the cytochromes in fish are most similar to the cytochromes in amphibians. But this is not the case.
And again:
To use the classic Darwinian scenario, amphibians are intermediate between fish and the other band-dwelling invertebrates. Analysis of their amino acids should place amphibians in an approximately intermediate position, but it does not.
(Note the use of Darwin’s name to denote the whole of evolution. In fact, the book is obsessed with Darwin, mentioning him on almost every page, and on some pages up to 10 times. In fact, by my count, and I may have missed a few, Darwin’s name or some variant of it occurs 262 times within the 144 pages of text. This emphasis on Darwin is of course found throughout the whole of the creationist literature, although by now evolutionary theory is almost as different from what Darwin proposed as atomic theory is from that proposed by Democritus.)
These are just two of five separate reiterations of the fallacy, leading up to the extraordinary statement that
Based upon the evolutionary series, we should expect some amphibians to be closer to fish (“primitive” species) and others to be closer to reptiles (“advanced” species).
And to make sure that the message sticks, we have this Figure, with the plain implication that the data point, not to evolution, but to separate creation:
The fallacy is not merely being stated; it is being repeated, rationalised, and reinforced. The kindest explanation is that the authors simply do not understand the science that they are presenting, seeing a hierarchical structure where none exists, and imposing on their biology a perspective in terms of “higher” and “lower” which do not belong in modern science, but have been carried over, such as the power of human vanity, from a worldview more akin to Aristotle and the mediaeval Great Chain of Being. The same fallacy also occurs in Michael Denton’s 1985 Evolution – A Theory in Crisis, and while he had by 1998 [3] quietly walked away from this, his 2016 sequel, Evolution – Still a Theory in Crisis, retains his preference for Aristotelian over phylogenetic classification.
And why should this matter? Because it reminds us, and we should not forget, that the Discovery Institute does not only deal in dis-information, but in dis-education.
I thank Maarten Boudry, Glenn Branch, Joe Felsenstein, John Harshman, Kim Johnson, Larry Moran, and Massimo Pigliucci for helpful comments and links to the literature.
Footnotes and citations:Permalink
(1) Dean Kenyon (co-author), Charles Thaxton (Academic Editor), and Stephen Meyer, Michael Behe, and Nancy Pearcey (contributors) all hold positions at the Discovery Institute, as do Raymond Bohlin, Walter Bradley, Robert Kaita, J.P. Moreland, and Paul Nelson, who are on the list of those thanked for being “critical reviewers”, as, also, are Meyer and Behe.
(2) Walter M Fitch and Emanuel Margoliash, Science 155(3760), 279, 1967; DOI: 10.1126/science.155.3760.279
(3) For a discussion of Denton’s revised position, see this 2006 post at Larry Moran’s Sandwalk blog
Repost of https://pandasthumb.org/archives/2023/01/Pandas-and-Frogs.html
Answers in Genesis appoints dangerous climate change denier as Chief Ministry Officer

“Though some weather events result from conditions on a fallen earth, Scripture is quite clear that God is in control.” This carefully crafted tweet by Answers in Genesis a few days ago is almost certainly the work of their newly appointed Chief Ministry Officer, Martyn Iles. Since God is in control, human activity cannot be responsible for the state of the planet, and the suggestion that we should adjust our policies because of their global impact is not only misguided, but impious.
Temperatures may be higher than at any time since the origin of humankind, ice caps may be melting in Arctic and Antarctic, and the smoke from forest fires may be making the air in New York unbreathable, but all of that is beside the point, because such things are to be expected on a fallen earth. The underlying reason for global warming is not fossil fuel burning, but Adam and Eve eating the forbidden fruit in the Garden. Thus the entire corpus of scientific evidence and climate observation is pre-emptively dismissed as irrelevant.
Notice that Iles is, despite the headline, not actually a climate change denier. He simply bypasses the question of whether climate change is happening, in order to move directly to the conclusion that if it is, we shouldn’t be trying to do anything about it. Because God. Such sophistical subtlety is central to his impressive rhetorical technique.
It would be wrong to dismiss such thinking as lunatic fringe. Lunatic, yes, but fringe, in the context of both Australian and North American politics, anything but. Over the past 20 years, a strong alliance has emerged, with its own special pseudoscience as justification, between young earth creationism, climate change denial, and conservative politics. There are, in the US at least, direct links between the creationist ministries, the fossil fuel industry, and influential right-wing think tanks. The alliance between evangelicals and climate change deniers played a major role in the election of Scott Morrison, Australian Prime Minister until last year, and of Donald Trump, both of whom did everything they could to block attempts to control carbon dioxide emissions. Similar alliances are at work in Alberta, and in Texas where the legislature is placing obstacles in front of the emerging renewables energy industry, despite its massive contributions to the State’s economy.
Martyn Iles, a lawyer by training, has been a major force in Australian religious politics. He was the managing director of the Australian Christian Lobby (ACL) from 2018 until he was abruptly sacked by the ACL Board in February 2023. Accounts of his dismissal differ. Iles described it as a result of difference in strategy; the Board wanted to move in a more political direction, making him in his own words “not the right person for that vision. I have always been a preacher first and politician second (or third…)”. The Board’s chair, however, denied that there had been any such change.
AiG is the property of Ken Ham, like Iles a product of Australian’s extreme Christian fundamentalist community. It was set up in 1994 after complex and litigious manoeuvres involving Ham and his previous associates, Creation Ministries International based mainly in Australia, and the Institute for Creation Research (ICR). ICR itself had been set up by Henry Morris, co-author of The Genesis Flood, when disputes arose among an earlier generation of Young Earth creationists.
There comes a time in the life of every successful businessman (it usually is a man) when he starts to consider his legacy. Ham is now 71. The vigour of his early writing, which had attracted Henry Morris’ attention in the 1980s, has faded into stale repetitiousness, and his articles on the AiG website now describe themselves as produced with the help of research staff. It seemed at one time as if Bodie Hodge, his son-in-law, was his obvious heir apparent, but Hodge’s own writing is superficial and tedious. (Disclosure; both Ham and Hodge have attacked me by name in their writings.)
Iles is now, therefore, in an extremely strong position within the organisation, for which he has excellent credentials. He is a successful organiser and money raiser, and responsible for targeted interventions in Australian electoral politics. His Youtube series The Truth of It has a major following, and as we shall see is very good at what it does. Thus we can expect him to be a major influence on AiG in its direction and messaging, and to enhance its appeal and effectiveness. He has already been announced as a key speaker in next year’s homeschooling conference.
It is thus a matter of some general concern that Iles is an extreme religious conservative, defines reality itself in religious terms, believes in male domination (while I was preparing this piece he told us that “A word like ‘independent’ is a direct assault on God’s design for women” and that a good woman is “Submissive to husbands. including imperfect ones”), is adept at promoting an intolerant agenda in the name of freedom of speech, has (ever so obliquely) inflamed concerns about vaccines, takes the historical truth of the early chapters of Genesis for granted, and thinks abortion should be illegal because God approves of population growth, among other reasons. Worst of all, he preaches that Christians must dismiss the findings of climate change science as “cultural Marxist rubbish,” because “God’s sustaining providence is crucial to our understanding of this world.”
For an example of Iles defending the indefensible, provided that the indefensible is based on religious belief, see his condemnation of Covid vaccine mandates.
To see him in unrestrained conspiracy mode, watch [1] his response to the World Economic Forum’s concept of a Great Reset, according to which we should use the pause imposed by Covid to rethink current industrial policy and its large-scale environmental impact. This notion offends against his core belief that the planet is in God’s hands, so that WEF’s concerns are fundamentally misguided. Like others, he presents the Reset concept, and the interest shown in it by governments, institutions, and major companies, as a conspiracy to do away with capitalism and democracy. Here, Iles is in lockstep with the Heartland Institute, a mouthpiece for the fossil fuel industry and for laissez-faire economics. As a sign of this conspiracy (and here I am reminded of Q-Anon) he points to the way in which the slogan Build Back Better, which occurs in the WEF literature, is echoed by politicians as diverse as Joe Biden, Boris Johnson, and Justin Trudeau, while as co-conspirators he identifies the entire climate change movement, as well as Black Lives Matter which, like other creationist writers, Iles describes as Marxist.
Iles’ full talents are on display in his The Truth of It YouTube, Climate Totalitarianism, which I recommend to students of rhetoric. Its thousand closely argued words are a masterpiece of misdirection, false dichotomy, strawmanning and vilification of opponents’ positions (the word cancer occurs four times); emotional engagement with the concerned, leading to a promise of reassurance and erasing of anxiety; imposing an intellectual superstructure (which he calls hierarchies of control) on the Bible and then using this superstructure to argue that mere worldly science can be safely ignored; slyly referring to fossil fuels by another name (mineral resources) as put there by God for humanity to use; and hinting at massive totalitarian conspiracies behind climate policy. All reinforced by dramatic phrasing, intonation, and gestures.
The title of the series, The Truth of It, prepares us for the message that anyone Iles disagrees with has been misleading us. The individual podcast title, Climate totalitarianism, casts the entire climate issue in terms of individual freedom versus governmental overreach, echoing his recurrent motif of a conspiracy of the powerful against the godly. And his opening sentence, “Well, it looks as if in the post-pandemic world, we’re going to be increasingly preoccupied with climate change,” describes a crisis over 50 years in the making as if it was just the next thing that they want us to worry about.
Iles then gives us two examples of net zero policy in action. Firstly, the enforced shutdown of Netherlands farms, early victims of the climate juggernaut (“there will be more”). I can find no reference to these alleged closures; the most relevant EU document that I could find sought, on the contrary, to reduce the loss of farmland, but no matter; our sympathies have been engaged with the alleged victims of the juggernaut, as have our fears, since we may be next. Secondly, eating bugs rather than red meat. Clearly, the net zero policy is unnatural, disgusting, and destructive.
Where do such misguided policies come from? From evolutionary thinking, of course. “I understand why they’re getting it wrong, because they basically believe that human beings arose on this planet quite by chance, and in time proceeded to go on a destructive, and a murderous, and exploitative, and a cancerous rampage, which must now be stopped.” (The word “cancer,” in connection with any concerns about human impact on the planet, occurs three more times in this presentation.)
If only our decision-makers would pay proper attention to the Bible! There they would find (Iles gives chapter and verse) that the descendants of Adam, and the descendants of Noah, were commanded to be fruitful and multiply, that Adam and his descendants were given dominion over everything on earth, and that God promised Noah that springtime and harvest would never cease as long as the Earth endures. Those who are worried about climate change have failed to recognise the hierarchy of control, according to which the planet was created to be adequate to human needs. It is humanity’s right, and indeed duty, to get to work and enjoy what has been made available, in the secure knowledge that caring for the planet as a whole is not their responsibility, but God’s.
Notice here the construction of a vast theological superstructure on a narrow biblical foundation, followed by the claim that this superstructure is itself biblical.
Like a judo player, Iles now uses the very force of the environmental argument as a reason for rejecting it. “If I thought we were here by chance, and we were just one of the gazillions of planets and we were just very fortunate to be in the position that we are in, I would think the future was pretty uncertain, and I’d get pretty nervous.”
Fear not. This nervousness is dispelled if we remember the hierarchy of control, and what God has promised: “Genesis is quite clear that what we see in the world around us was substantially put there for human use, and enjoyment, and sustenance, including plants, water, minerals, and animals.” The word minerals is the only reference in the piece to fossil fuels, but its significance will not be lost on his intended Australian primary audience.
Governments pursuing environmental goals are in an extremely stressful situation, he tells us, since they are going against fundamental human nature, and must use totalitarian methods to impose their will. But this stress is unnecessary, if we remember the divinely ordained hierarchy. Humankind is steward of the planet, but God is an even greater steward, and we should listen to His word.
The most alarming part of Iles’ sermon is what he does not say. He simply bypasses the scientific evidence that business as usual risks unacceptable damage to the environment. Implicit in his position is the acceptance that such things, if they happen, will represent the working out of God’s will.
For those who see us as approaching the End Times, as I suspect Iles does, this is merely spelling out the obvious. For the rest of us, terrifying.
I thank Dan Phelps for useful background information about AiG’s empire, and the Rev Michael Roberts for helpful comments. Earlier versions of this material Appeared on Panda’s Thumb and 3 Quarks Daily.
1] Disclosure. Life is short, so once I’ve got the flavour of a presentation, I just scan the transcript.
Repost of https://3quarksdaily.com/3quarksdaily/2023/06/leading-creationist-organisation-appoints-conspiracy-theorist-to-key-position.html and https://rightingamerica.net/leading-creationist-organisation-appoints-conspiracy-theorist-to-key-position/
Explosives, Fertilisers, Chemical Weapons and the Unintended Consequences of Discovery: The Tragedy of Fritz Haber
What’s a tragedy? Hamlet is a tragedy, not just because our hero ends up dead, taking half a dozen people with him, but because his own reflective intelligence is instrumental in his fate. By this strict definition, the story of Fritz Haber, indicted war criminal, Nobel laureate, patriot and miserable exile, is indeed a tragedy. He sought to serve this country, and helped destroy it. The moral dilemmas of Haber’s career will not go away, and the ironies of unintended consequences are timeless. [Notes on talk to Callander and W Perthshire U3A, 22 March 2023]
Sources
- Einstein’s German world, Fritz Stern
- Fritz Haber – Chemist, Laureate, German, Jew, Dietrich Stoltzenberg
- (See also Enriching the Earth: Fritz Haber, Karl Bosch, and the Transformation of World Food Production, Vaclav Smil)

BBC muzzles David Attenborough, suspends top sports presenter, for fear of Tory backlash
by Paul Braterman
UPDATE (March 15, 2023): The Home Secretary has said in the House of Commons that “Police chiefs around the country, what they tell me is that criminality, in particular drug supply and usage, is now connected to people who came here on small boats illegally in the first place.” WhatDoTheyKnow has made a Freedom of Information request to the Home Office, asking for evidence to back up this inflammatory claim.
The Guardian has revealed details of internal BBC emails in which a senior editor was asking reporters to modify their presentations because of complaints from Downing Street.
There will no doubt be more in the coming days, but I’ll leave it at that.
UPDATE (March 13, 2023, 8 AM): Gary Lineker has been reinstated, and has reaffirmed his position:
Read the rest of this entryA final thought: however difficult the last few days have been, it simply doesn’t compare to having to flee your home from persecution or war to seek refuge in a land far away. It’s heartwarming to have seen the empathy towards their plight from so many of you.
Why a key creationist climate change denier has gone antivaxx
Summary: The Cornwall Alliance for the Stewardship of Nature presents itself as a Christian thinktank on environmental ethics. In reality, it is a direct link between evolution denial and climate change denial, with personnel overlapping Answers in Genesis, and
direct links to the Heartland Institute, a mouthpiece for the fossil fuel industry, and the influential Heritage Foundation. It is now engaged in assembling an ideological package, based on rejection of the principle that policy should be guided by scientific knowledge, and linking together everything from evolution to environmental concerns to elementary measures for restricting the spread of Covid. The rhetoric is masterly; the consequences, lethal.
A friend just sent me a copy of materials that the Cornwall Alliance is sending to its supporters. Here is an extract [fair use claimed]:
BE ARMED AGAINST THE DANGERS OF SCIENCE SO CALLED
Question any part of the climate-change “consensus” (how much climate change is going on, how much humans contribute to it, what if anything we should do about it), and you’re instantly declared “anti-science” or even a threat to the future of the human race.
But don’t be intimidated—or fooled. That response is itself anti-science. It is rhetoric designed to win not by persuading others but by silencing them.
And it arises not just about climate change. From good old Darwinism (goo to you by way of the zoo) and Malthusianism (population growth inexorably exceeds food production and causes a sudden die-off), to the Obama Administration’s insistence that employers must provide insurance coverage for contraception and abortion regardless of their religious conscience, and COVID-19 mask, social distancing, travel, church worship, and vaccine policies.
People in America and around the world are in danger of becoming slaves of scientism and scientocracy.
The rest of the piece is a blurb for an essay by John G West that forms part of a forthcoming book on CS Lewis and his views on the relationship between science and religion (science ought to know its place), leading up to an appeal for funds. The Cornwall Alliance is a charity under US law, rather than a political body, and contributions are tax-deductible.
Read the rest of this entryBlack swans and other deviations: like evolution, all scientific theories are a work in progress

Discussions about the nature of science and scientific theories are often confused by the outdated view that such theories are rendered false when anomalies arise. The notion of a scientific theory as a static object should be replaced with the more current view that it is part of a living research programme, which can broaden its scope into new areas.
For example, take the hypothesis that all swans are white, which seemed pretty good to Europeans until Dutch explorers found black swans in Australia in 1636. So what happens to our hypothesis? There are a number of options.
1) Redefine swan-ness to include whiteness. Then black swans aren’t really swans, and the hypothesis remains true by definition.
2) It’s been disproved. Discard it.
3) Compare different species of swan the world over, and see how well black swans fit in.
(1) is the least useful. Definitions can only tell us about how we are using words. They tell us nothing about the world that those words attempt to describe. (2) is based on the common-sense idea that hypotheses should be discarded when falsified by observation. This was the idea put forward by philosopher Karl Popper in the 1930s, to distinguish between science and pseudoscience.
Read the rest of this entrySensory Worlds Beyond Our Imagining
An Immense World; How Animal Senses Reveal the Hidden Realms Around Us, Ed Yong, Random House/Bodley Head, June 2022
This book is an enormous achievement. A thrilling read, taking us into the Umwelt, or perceptual world, of numerous mammal, fish, reptile and insect species. A major work of scholarship, with over a thousand references to a 45-page bibliography, as well as accounts of interviews with numerous researchers and visits to their laboratories. An exploration of many ways of sensing the world, some of which we share, while others are beyond our imagining. The evolving interplay of perception and action, communication and deception, environment and response. And an enhanced insight into what it is like to be a bat, a bird, a blue whale, a beetle, or a human.
From the wealth of detail in the book, a consistent grand narrative emerges. Some physical process interacts with living matter. This is the raw material for sensation. Sensory abilities then shape a creature’s Umwelt, being developed according to the demands of its environment. But every perceiver is itself an object of perception to others, and we have colour displays and camouflage, smells as signals and identifiers, sound as communication to others and, by echolocation, back to the creature who generates it, and the same is true of other senses that we do not share, such as the detection of tiny electrical fields. Senses combine and even, we suspect, merge, and what we ourselves perceive is but part of an immense pattern. But the heedlessness with which we amplify our own signals disrupts this pattern, contributing to our destruction of nature, and we ourselves are the poorer for it.
Let me offer a few samples from the book’s wealth of detail.
Yong starts with taste and smell, two ancient senses that operate by direct molecular contact. It is not long before he surprises us. Snakes use their forked tongues to smell in stereo. Humans are poor compared with other mammals at detecting smells at low levels, but are rather good at telling different smells apart. No one knows how smell relates to chemical structure (contrast this with how seeing relates to the wavelength of light, hearing to frequency, or touch to pressure). As every well-trained dog-owner knows, smell is central to the Umwelt of dogs, but I would never have guessed that the same is true of elephants. And the molecules involved in smell include opsins, which are central to vision. As Yong puts it, in a way we see by smelling light.
Read the rest of this entryEvolution 101, with reference to coronavirus
By T. Ryan Gregory. Reproduced with permission
Mutations occur as chance errors in replication. They’re just mistakes in copying. Most have no effect. Some are detrimental to the organism (or virus), a few may happen to be beneficial — this depends on the environment.
Three main evolutionary processes determine what happens to genetic variation once it arises (and these are independent of the process that generates new variation, namely mutation): genetic drift, natural selection, and gene flow.
We call different versions of a gene “alleles” and we can talk about the proportion of those versions in a population as “allele frequencies”. Genetic drift is a random change in allele frequencies that occurs by chance.
Genetic drift is basically sampling error, in which the genetic variation in a new generation does not accurately reflect what was present in the previous generation. The most obvious mechanisms of genetic drift are founder effects and population bottlenecks. (But see below about genetic drift being common in small populations generally). Founder effects occur when a random, non-representative subset of a population moves to a new location and founds a new population. The allele frequencies in that founder population won’t be the same as in the source population, and there will typically be less variation overall.
Population bottlenecks are sudden, severe declines in population size in which survival happens at random. So, for example, a drought or storm causes a major die-off and the individuals who survive were just lucky (rather than having traits that helped them survive).
Gene flow is movement of genetic variation from one population to another. The overall effect is to introduce new variation into an existing population (if the source population has different alleles) and to make two populations that exchange alleles more similar to each other.
When we think about the early stages of new species evolving, we generally are considering ways that gene flow is being blocked. Lots of gene flow means two populations are less likely to diverge genetically.
The final evolutionary mechanism is natural selection. In this case, the reason some individuals survive and reproduce better than others is *non-random*. It doesn’t occur by chance. It is specifically related to heritable traits that make survival and reproduction more likely.
This is where the concept of “fitness” is relevant. In evolutionary terms, fitness refers to the advantage in survival and/or reproduction due to heritable traits. Fitness depends on the environment. What is fit in one environment may be neutral or unfit in another environment.
There are different forms of natural selection, depending on what part of the distribution of traits is fit/unfit in the population: directional selection, diversifying (or disruptive) selection, and stabilizing selection.
Under directional selection, one extreme of the distribution is fit and the other is unfit. This drives the distribution of traits in a particular direction from one generation to the next.
For example, if the largest individuals leave more offspring on average than smaller individuals in each generation, then the average size will increase over time. Not because individuals start being born larger in response, but because more offspring are born of large parents.
In diversifying selection, it is the two extremes that are fit and the average traits that are unfit. This can cause a population to split into two. For example, if the smallest and largest individuals do well but the medium-sized are at a disadvantage.
Finally, in stabilizing selection the average value is fit and the extremes are both unfit. The result is that this prevents the distribution of traits from changing in the population because deviations from the current average are detrimental in that environment.
I have added an image from Wikipedia to show these three types of natural selection. A is the original distribution of traits, B is the new distribution. 1) Directional, 2) Stabilizing, 3) Diversifying selection.

There are many factors that affect these evolutionary mechanisms. Mutation rates can be high if there is weak quality control and repair of errors, or if there is some environmental factor (mutagen) that messes up replication.
It also matters how much replication is happening. Every time a genome is replicated, there can be errors. Lots of replication means lots of opportunities for mistakes to occur. (In multicellular organisms, only mutations in the germline are relevant in evolution, of course).
As to what happens to alleles, this depends on the environment as well as population size. Genetic drift, which is sampling error, is stronger when samples (i.e., populations) are smaller. Natural selection, which is non-random, is stronger when populations are large.
Whether an allele is fit or unfit (will be subject to non-random natural selection) or neutral (will evolve by random genetic drift) depends on the environment.
Natural selection and genetic drift can happen at multiple levels. The main one is, of course, among organisms within populations, but these can also happen within organisms. Cancer is an example of cell-level selection that is usually suppressed in multicellular organisms.
When it comes to viruses, there are two levels as well: within hosts and among hosts. Because viruses mutate so quickly (by chance, because their repair mechanisms are weak), there can be new variation arising within a single host.
Some mutants will do better within the host — that is, they will be better at invading host cells or will be replicated more quickly than other versions of the virus within a host.
So, there is natural selection within the host.Some mutants will do better at getting into new hosts. For example, maybe they form smaller aerosol particles and spread father when sneezed out. Or maybe they are in high concentration in the nose rather than deeper in the lungs, so they get shed more easily.
The mutant viruses that do best within a host are not necessarily the same ones that do better at infecting new hosts. In fact, a highly virulent version might be very effective at invading host cells but do so much damage that the host never spreads it to another host.
There can be a trade-off between virulence (replication within a host that causes damage to the host) and transmissibility (spread to new hosts). Which versions of a virus evolve depends on the mutations that happen to occur by chance replication errors and the outcome of genetic drift and natural selection both within hosts and among hosts.
Whether viral evolution involves increased or decreased virulence and/or higher or lower transmissibility depends on many factors. Number of replication events happening. Rates of replication errors. Selective pressures within and among hosts. Viral and host population sizes.
Virulence and transmissibility are not the same thing, and there may be trade-offs between them, but it’s also a concern that a virulent (damages or kills the host) virus can still be successful at the host population level if it is able to spread to many new hosts.
Viruses that are both highly virulent and transmissible will eventually run out of hosts to infect, but they can do great damage before that happens.
One of the many positive effects of reducing transmission (e.g., with vaccines, masks, etc.) is that this imposes a selection pressure for less virulence. If only versions of the virus that don’t incapacitate or kill the host manage to reach new hosts, then those are fitter. Reduced transmission also means fewer replication events happening and this means fewer new mutations.
A mild but highly transmissible version of a virus can spread quickly through a population and then fizzle out as hosts become immune, and many people seem to be assuming this will happen with Omicron, but that also means a lot of replication and new mutations.
The Omicron variant in particular has many, many mutations specifically in the spike protein, which is one reason it is so much more transmissible and escapes previous immunity. And this may now be the starting point for new variants.
It is possible that Omicron is milder (than Delta, at least) and that it will infect pretty much everyone and that this will be a step toward SARS-CoV-2 becoming endemic (like flu, requiring seasonal vaccinations).
But it is also possible that Omicron may undergo more chance mutations that make it more virulent as well as highly transmissible. Then it spreading rapidly will mean many hospitalizations and deaths before it runs out of hosts. We do know it is still evolving.
Viruses don’t want anything. They just spread to new hosts or they don’t, and replicate effectively in hosts or they don’t. Mutation, genetic drift, gene flow, natural selection. There are many factors we can’t control, but there are some that we can. We really ought to try.

Tall el-Hammam; an airburst of gullibility; it gets worse
I shared the excitement when I read at https://www.nature.com/articles/s41598-021-97778-3 that
in ~ 1650 BCE (~ 3600 years ago), a cosmic airburst destroyed Tall el-Hammam, a Middle-Bronze-Age city in the southern Jordan Valley northeast of the Dead Sea

and that this event could have given rise to the biblical account of the destruction of Sodom and Gomorrah. Then I learned that the work was conducted by a group based on an unaccredited Bible college (Trinity Southwestern University, TSU), that the world’s leading authority on airbursts has denounced the claims as impossible, that eight separate major research groups have questioned the assumptions, reproducibility, and factual accuracy of related earlier work by the corresponding author, that there is an unusually active thread criticisng the work on PubPeer, and that Retraction Watch, which says that criticism has engulfed the paper, is in correspondence with the Chief Editor of the journal, part of the Nature group, where the work appeared.
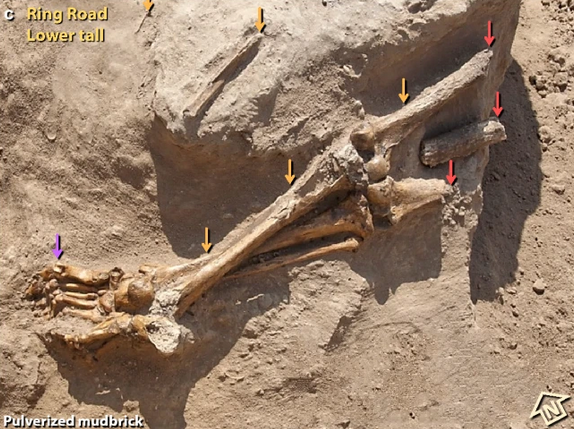
Problems listed by acknowledged experts in PubPeer include misuse of Mark Boslough‘s account of airbursts (Boslough is a long-standing critic of the claims of the TSU group); and no clear evidence that the destrution of the palace walls was catastrophic, absence of qualified examination of skeletons, anatomically misdescribed bones, claims without evidence that bone damage was associated with traumatic death, rather than later damage, the mixing of kinds of debris is commonplace and not evidence of catastrophe, the connection of the carbon-14 dates to the alleged destruction is not established, and claims of burning of bone lack evidence and consistency (Megan Perry of the Petra North Ridge Project, who knows what this kind of stuff looks like.) There is undeclared image manipulation, eventually admitted, but described as without significance. And the account of diamond vs graphite in the paper
Each diamonoid typically contains carbon atoms that are sp3-bonded (i.e., 3 bonded carbon atoms), as in diamond, rather than with sp2 bonding (i.e., 2 bonded carbon atoms), typical of graphite
does not inspire conidence.
In the paper’s Figure 44c, shadows are cast by a sun shining from the direction labelled North. This obviously cannot happen at Tall el-Hammam, giving rise to further concerns about the quality of the work.
My own browsing in what are for me odd places1 shows that other archaeologists, including Aren Maeir of Bar Ilan University, agree in finding nothing unusual in the report compared to “normal” destruction by fire or warfare) Maeir is quite explicit:
[T]he destruction the report described was not that unusual. “I see some things that remind me of phenomena that we have in the Iron Age IIA (1000–925 BC) destruction at Tell es-Safi/Gath (e.g. vitrified or “melted” bricks, ultra-high temperatures, and other things)—a destruction that is most likely caused by the conquest and destruction of the site by Hazael of Aram,” he said.
Mark Boslough, an expert on cataclysmic events who even has an asteroid named after him, tells me that he is tired of repeated rebuttal of what he considers to be obviously false claims, and of seeing his own theoretical analysis of the effect of airbursts invoked as an explanation of claims completely inconsistent with such a process, or perhaps any credible process. For this reason, rather than publishing yet another counterblast that will be ignored, he has taken to describing the controversy, extending over many years, on Twitter (see here, here, and subsequent threads, and PubPeer).
Phillip Silvia, the author from whom soil samples can be obtained, is an electrical engineer by training, who received his PhD training in archaeology at TSU, and published much of this material through TSU press in 2016 as a paperback, in which his PhD advisor, Steven Collins, strangely absent from the author list of this paper although not from an earlier abstract, states (Foreword, page x) that “the Genesis 19 description of Sodom’s destruction was about good a phenomenological description of a cosmic airburst as one could imagine” and describes Tall el-Hammam as “the site I had identified as Sodom based on geographical details embedded in the boblical text”.
Like I suspect many of the journalists and interested readers who swooped on this story, I failed at first to notice that the paper was not, despite the link, an article in Nature but in Scientific Reports. This is one of the stable of less exclusive journals closely linked to Nature being published by Springer, now controlled by the publishing giant Hotlzbrinck, and profiting from Nature’s reputation for excellence. I was only vaguely aware of the authors’ long history of invoking airbursts, took the many kinds of evidence listed at face value, and did not even blink at the claim that “[a]n airburst-related influx of salt (~ 4 wt.%) [from the Dead Sea, apparently] produced hypersalinity”.

The authors have since described this work to a much larger audience in The Conversation, where they repeat their claim, also published in Scientific Reports, of a similar catastrophe at Abu Hureyra in what is now Syria, around 10,800 BCE, assert that “it almost certainly won’t be the last time a human city meets this fate”, claim that such events “pose a severe modern-day hazard”, and advise that “unless orbiting or ground-based telescopes detect these rogue objects, the world may have no warning, just like the people of Tall el-Hammam.” The Abu Hureya paper also repeats a litany of earlier claims that the Younger Dryas, a period of severe cold in the northern hemisphere from around 12,900 to 11,700 years Before Present [Present is fixed at 1950 CE], was caused by a series of impacts with cometary debris, spread over at least four continents. These claims have been severely disputed; see papers listed below.
There are numerous additional reasons for concern about the TSU researh group.

The paper tells us that “The project is under the aegis of the School of Archaeology, Veritas International University, Santa Ana, CA, and the College of Archaeology, Trinity Southwest University, Albuquerque, NM, under the auspices of the Department of Antiquities of the Hashemite Kingdom of Jordan.” Veritas International University believes in “the full historicity and comprehensibility of the biblical record”. It is accredited by the Transnational Association of Christian Colleges and Schools (TRACS), which I have discussed here before. Trinity Southwest University, which now operates from an office in a strip mall in Albuquerque, was formerly in Tulsa, Oklahoma, under the name Southwest Biblical Seminary, and rejects any government accreditation whatsoever as intrusive violation of the separation of Church and State. While the traditional site of Sodom is in Israel (and within the pre-1967 boundaries).

The corresponding author is Allen West, who has been publishing prolifically in this area since 2005 (Evidence for the Extinction of Mammoths by an Extraterrestrial Impact Event) and in 2006 co-authored a book, The Cycle of Cosmic Catastrophes: How a Stone-Age Comet Changed the Course of World Culture, which claims that the debris of a shattered comet was responsible for “a cosmic chain of events [that] began 41,000 years ago and culminated in a major global catastrophe 28,000 years later.” These events include everything from the extinction of the mammoths to the landform of the Carolina Bays to the legend of Atlantis to a purported “mysterious layer of black sediment” found spanning North America to the Younger Dryas discussed in the Abu Hureya paper. West has no academic qualifications or affiliations, and gives his address as Comet Research Group (CRG), Prescott, Arizona (several of the other authors are also members of this group, which is linked to the Rising Light Group, a 501(c)3, tax-exempt charitable organization with a clear Christian and biblical agenda, registered in Allen West’s name.). As detailed by Pacific Standard Magazine, discussing how thing stood regarding CRG’s work in 2017, there have been calls for a for a formal inquiry and
University of Wyoming archaeologist Todd Surovell and his colleagues couldn’t find increased magnetic spherules representing cosmic debris at seven Clovis sites. Nicholas Pinter and his colleagues at Southern Illinois University Carbondale argue the carbon spherules are organic residue of fungus or arthropod excrement. And Tyrone Daulton of Washington University in St. Louis and his colleagues reported that supposed nanodiamonds formed by the impact were misidentified.
On the other hand, in an acrimonious exchange with me in the Comments section of The Conversation, West pointed out that
Our group included, among many others: Dr. James Kennett, emeritus professor at UCSB, specializing in stratigraphy, micropaleontology, paleobiology. He is a member of the National Academy of Sciences, which recognizes the contributions of just 0.1% of all scientists. Dr. Ted Bunch, former NASA section chief and a world-leading meteoriticist. Dr. Robert Hermes, retired from Los Alamos National Labs, world-recognized expert in trinitite or atomic glass. Dr. Wendy Wolbach, chemistry professor who discovered high-temperature soot at the K-Pg boundary.
I replied with a listing of some papers that I have examined criticising West’s own earlier work regarding airbursts, including sampling techniques and claimed evidence for very high temperatures:
Scott AC, Hardiman M, Pinter N, Anderson RS, Daulton TL, Ejarque A, Finch P, Carter-champion A (2017). “Interpreting palaeofire evidence from fluvial sediments: a case study from Santa Rosa Island, California, with implications for the Younger Dryas Impact Hypothesis”. Journal of Quaternary Science. 32 (1): 35–47. doi:10.1002/jqs.2914.
*Boslough M, Harris AW, Chapman C, Morrison D (November 2013). “Younger Dryas impact model confuses comet facts, defies airburst physics”. Proceedings of the National Academy of Sciences of the United States of America. 110 (45): E4170. doi:10.1073/pnas.1313495110.
*Boslough M (April 2013). “Faulty protocols yield contaminated samples, unconfirmed results”. Proceedings of the National Academy of Sciences of the United States of America. 110 (18): E1651. doi:10.1073/pnas.1220567110
Van Hoesel A, Hoek WZ, Pennock GM, Drury MR (2014). “The Younger Dryas impact hypothesis: a critical review”. Quaternary Science Reviews. 83: 95–114. doi:10.1016/j.quascirev.2013.10.033.
*Meltzer DJ, Holliday VT, Cannon MD, Miller DS (May 2014). “Chronological evidence fails to support claim of an isochronous widespread layer of cosmic impact indicators dated to 12,800 years ago”. Proceedings of the National Academy of Sciences of the United States of America. 111 (21): E2162-71. doi:10.1073/pnas.1401150111.
*Holliday VT (December 2015). “Problematic dating of claimed Younger Dryas boundary impact proxies”. Proceedings of the National Academy of Sciences of the United States of America. 112 (49): E6721. doi:10.1073/pnas.1518945112.
Thy P, Willcox G, Barfod GH, Fuller DQ (2015). “Anthropogenic origin of siliceous scoria droplets from Pleistocene and Holocene archaeological sites in northern Syria”. Journal of Archaeological Science. 54: 193–209. doi:10.1016/j.jas.2014.11.027.
*Van der Hammen T, Van Geel B (2016). “Charcoal in soils of the Allerød-Younger Dryas transition were the result of natural fires and not necessarily the effect of an extra-terrestrial impact”. Netherlands Journal of Geosciences. 87 (4): 359–361. doi:10.1016/j.jas.2014.11.027
See, however, in defence of the Younger Dryas impact theory, *Sweatman MB (2021). The Younger Dryas Impact hypothesis: Review of the impact evidence. Earth-Science Reviews. 218: 103677. doi:10.1016/j.earscirev.2021.103677. I thank Christopher R. Moore, one of the authors of the paper I am criticising, for drawing my attention to this review.
*Freely accessible via doi; for other papers, doi gives access to abstracts but not full text.
We await further developments with interest.
1] Bit for a rebuttal by a biblical archaeologist involved in the dig, though not an author on this paper, see here
An earlier edition of this post appeared on pandasthumb.org